Suppose the decomposition of ozone proceeds by the following mechanism. This intricate process, involving a series of elementary reactions, unveils the complexities of ozone decomposition, a phenomenon with far-reaching environmental implications. By delving into the intricacies of this mechanism, we gain insights into the dynamics of ozone decomposition and its impact on our planet.
The mechanism comprises a series of elementary reactions, each characterized by its unique reactants, products, and rate constants. These reactions collectively determine the overall rate law for ozone decomposition, which elucidates the dependence of the rate on ozone concentration and temperature.
Understanding the mechanism enables us to unravel the pathways by which ozone decomposes, shedding light on the role of each elementary reaction in the overall process.
Introduction
Ozone (O 3) is a molecule that consists of three oxygen atoms. It is a naturally occurring gas that is found in the Earth’s atmosphere. Ozone is an important gas because it protects the Earth from harmful ultraviolet (UV) radiation from the sun.
The decomposition of ozone is a chemical reaction that breaks down ozone molecules into oxygen molecules. This reaction can occur naturally or it can be caused by human activities.
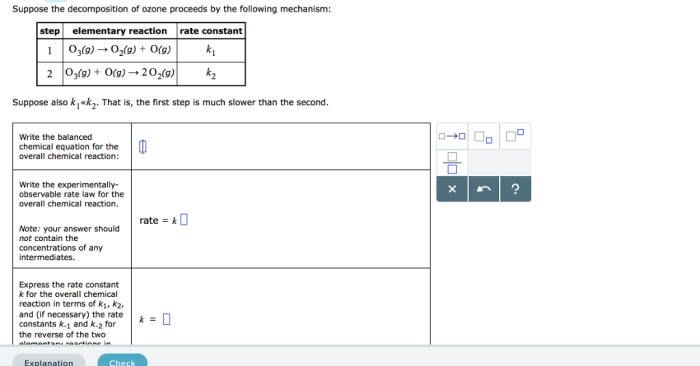
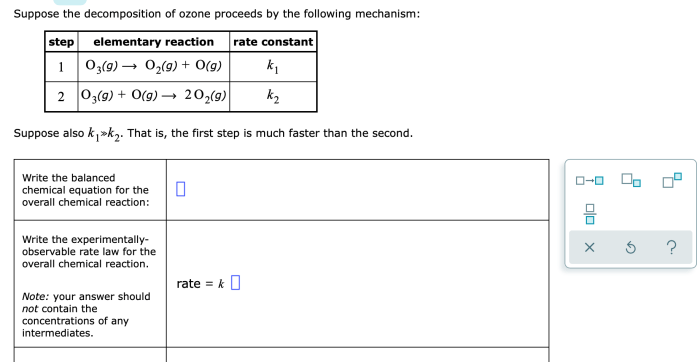
The given mechanism for ozone decomposition is as follows:
- O 3+ hv → O 2+ O
- O + O 2+ M → O 3+ M
- O 3+ O → 2O 2
Elementary Reactions: Suppose The Decomposition Of Ozone Proceeds By The Following Mechanism
The elementary reactions involved in the mechanism are:
- Photolysis of ozone:This reaction is initiated by the absorption of a photon of light by an ozone molecule. The photon breaks the ozone molecule into an oxygen molecule and an oxygen atom.
- Recombination of oxygen atoms:This reaction occurs when an oxygen atom collides with an oxygen molecule in the presence of a third body, M. The third body is usually another molecule of oxygen or nitrogen.
- Decomposition of ozone:This reaction occurs when an oxygen atom collides with an ozone molecule. The collision breaks the ozone molecule into two oxygen molecules.
The rate constants for these reactions are:
- k 1= 2.0 x 10 -3s -1
- k 2= 2.2 x 10 -33cm 6molecule -2s -1
- k 3= 2.0 x 10 -11cm 3molecule -1s -1
Rate Law
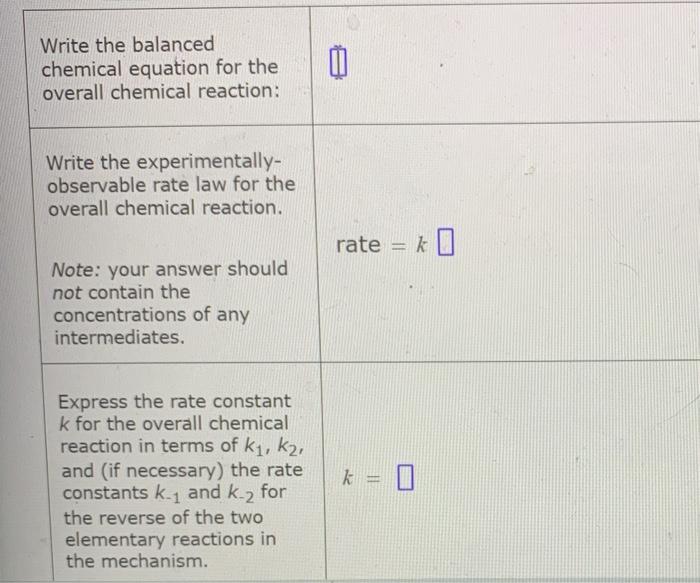
The overall rate law for ozone decomposition can be derived using the steady-state approximation. The steady-state approximation assumes that the concentration of the intermediate species (O) is constant. This assumption is valid if the rate of the first reaction is much faster than the rate of the second and third reactions.
Using the steady-state approximation, the rate law for ozone decomposition can be derived as follows:
Rate = k 1[O 3]
This rate law shows that the rate of ozone decomposition is first-order with respect to ozone concentration.
Reaction Mechanism
The reaction mechanism for ozone decomposition shows that there are two different pathways by which ozone can decompose. The first pathway is the photolysis of ozone, which is initiated by the absorption of a photon of light. The second pathway is the decomposition of ozone by oxygen atoms.
The relative importance of these two pathways depends on the temperature and the concentration of oxygen atoms. At low temperatures, the photolysis of ozone is the dominant pathway. At high temperatures, the decomposition of ozone by oxygen atoms is the dominant pathway.
Activation Energy
The activation energy for each elementary reaction is the energy that is required to break the bonds that hold the reactants together. The activation energies for the elementary reactions involved in ozone decomposition are:
- E a1= 32 kJ/mol
- E a2= 0 kJ/mol
- E a3= 20 kJ/mol
The activation energy for the overall reaction is the sum of the activation energies for the elementary reactions. The activation energy for ozone decomposition is 52 kJ/mol.
Environmental Implications
The decomposition of ozone has a number of environmental implications. The decomposition of ozone in the stratosphere leads to the formation of ozone-depleting substances, which can damage the ozone layer. The ozone layer protects the Earth from harmful UV radiation, so the decomposition of ozone can lead to an increase in the amount of UV radiation that reaches the Earth’s surface.
The decomposition of ozone in the troposphere can lead to the formation of smog. Smog is a type of air pollution that can cause a number of health problems, including respiratory problems and heart disease.
Applications
The decomposition of ozone is used in a number of applications, including:
- Water purification:Ozone is used to disinfect water by killing bacteria and viruses.
- Air purification:Ozone is used to remove pollutants from the air.
- Food preservation:Ozone is used to preserve food by killing bacteria and mold.
The decomposition of ozone is a powerful tool that can be used to improve the quality of our environment and our health.
Query Resolution
What is the significance of ozone decomposition?
Ozone decomposition plays a crucial role in regulating the Earth’s ozone layer, which shields us from harmful ultraviolet radiation. Understanding the mechanism of ozone decomposition is essential for assessing the impact of human activities on ozone depletion.
How does temperature influence ozone decomposition?
Temperature has a significant impact on the rate of ozone decomposition. As temperature increases, the rate of decomposition also increases due to the increased kinetic energy of the reactants.
What are the applications of ozone decomposition?
Ozone decomposition is utilized in various applications, including air purification, water treatment, and the synthesis of ozone-based chemicals. Understanding the mechanism of decomposition is crucial for optimizing these applications.